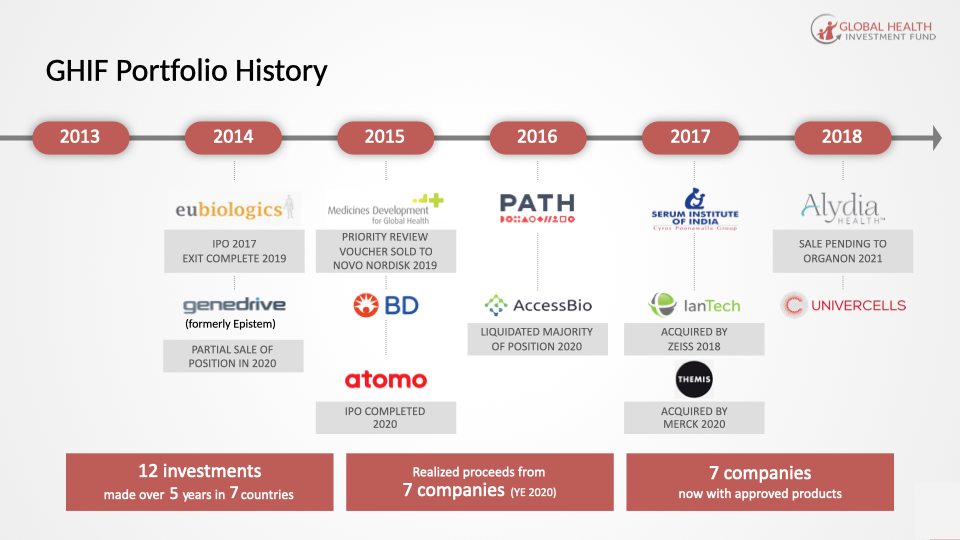
GHIF Portfolio Investments
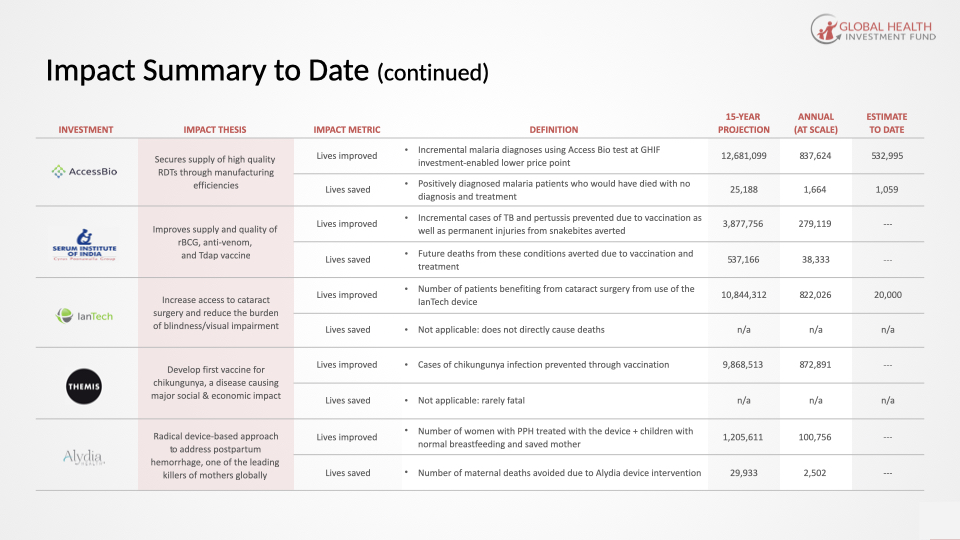
Intervention: Device
Targets: Postpartum hemorrhage
Impact Objectives: Develop new gold-standard intervention for PPH
Status: FDA Clearance 2020
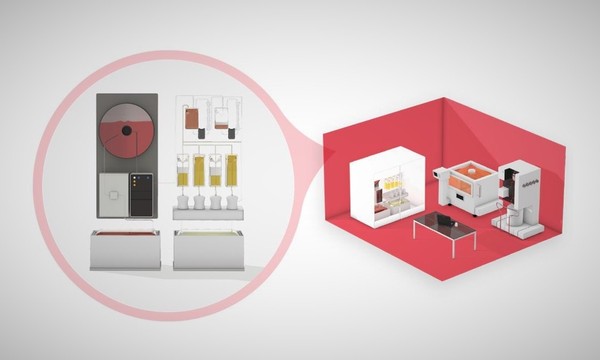
Intervention: Biologics manufacturing technology platform
Targets: Polio, Measles, Rubella, COVID-19
Impact Objectives: Dramatic reductions in the cost of essential biologics; distributed manufacturing
Intervention: Vaccines and therapeutics
Targets: Anti-venom for snakebites, vaccines for tuberculosis and pertussis
Impact Objectives: High quality anti-venoms, increased access to Tdap vaccines in pregnancy, more effective protection against tuberculosis
Status: Anti-venom approval in India pending, other products in clinical studies
Intervention: Vaccine platform
Targets: Chikungunya, Zika, COVID-19
Impact Objectives: Protect at-risk communities from public health challenges such as mosquito-transmitted viruses
Status: Acquired by Merck in 2020. COVID-19 and chikungunya vaccines in development
Intervention: Surgical device
Targets: Cataract-induced blindness and visual impairment
Impact Objectives: Expand access to vision-restoring cataract surgery in resource-limited settings
Status: Acquired by Zeiss. Product FDA cleared.
Intervention: Treatment
Targets: Hookworm, Roundworm, Whipworm
Impact Objectives: Cure hundreds of millions of school-aged children from parasitic worm infections; expand arsenal of anthelmintics
Status: Discontinued
Intervention: Diagnostic
Targets: Malaria, G6PDd, HIV, dengue, HPV, Zika, COVID-19
Impact Objectives: Reduced cost of high-quality malaria RDTs; new, low-cost diagnostics for other diseases
Status: >100 million malaria tests per year; COVID-19 antibody and antigen tests approved
Intervention: Diagnostic
Targets: HIV, Ebola, HCV, malaria, COVID-19
Impact Objectives: Improved professional testing and self-testing
Status: IPO April 2020 tests for HIV, COVID-19 and pregnancy on the market
Intervention: Diagnostic
Targets: Preeclampsia (PE), gestational diabetes mellitus (GDM)
Impact Objectives: Early, affordable, point-of-care PE/GDM testing; ↓ maternal/infant mortality/morbidity
Status: Discontinued
Intervention: Diagnostic
Targets: Tuberculosis (TB), HCV, COVID-19
Impact Objectives: Diagnosis of TB and HCV on a near-point-of-care molecular diagnostic platform, enabling more effective treatment
Status: Approved for HCV and COVID-19 tests
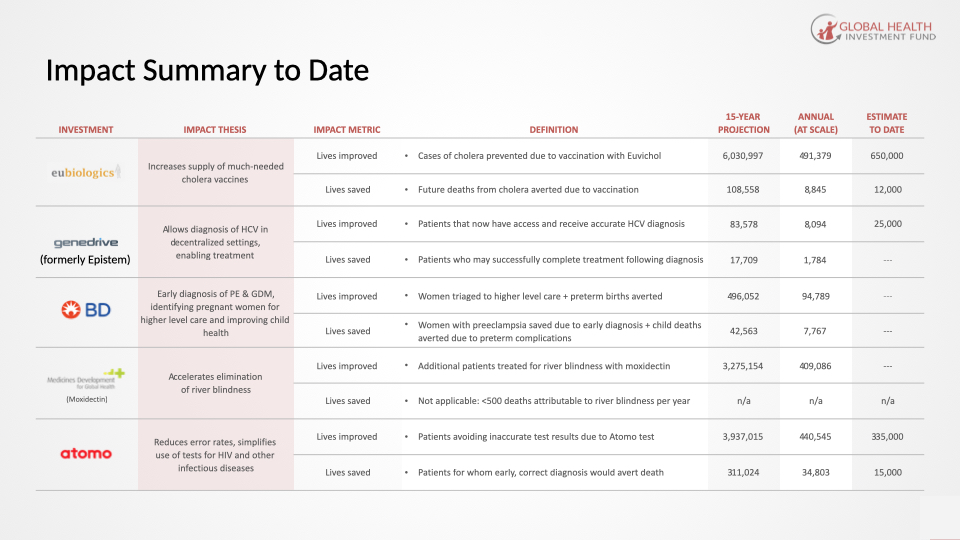
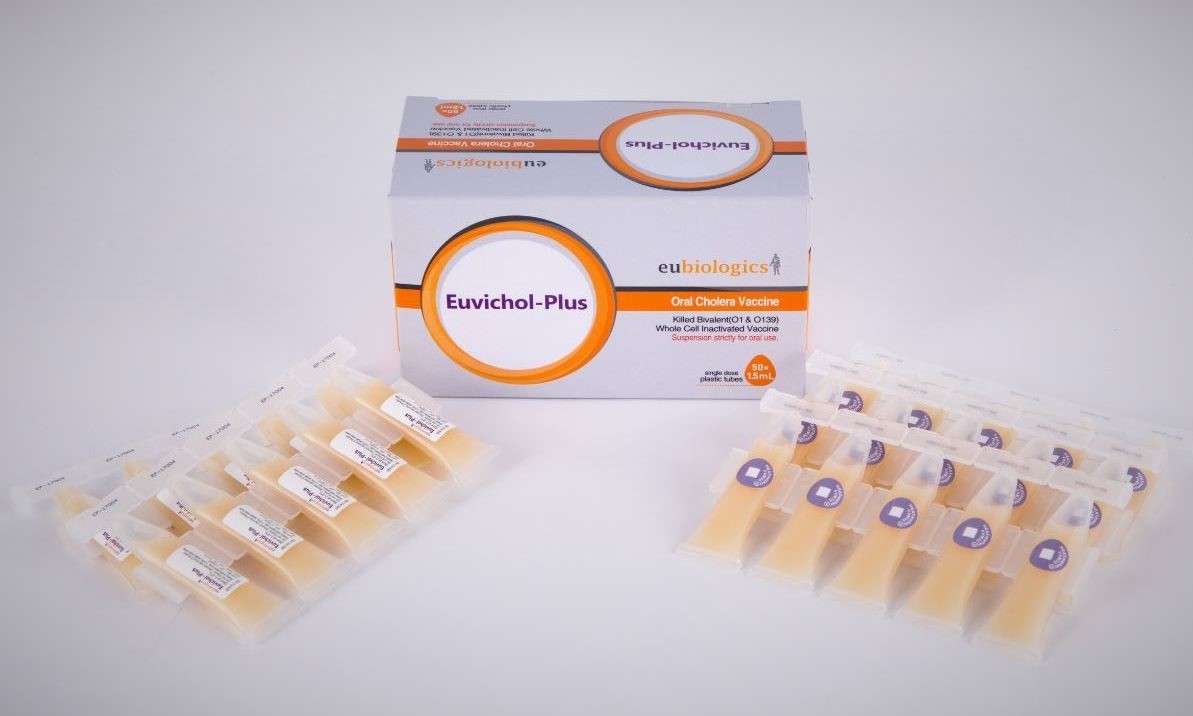
Intervention: Vaccine
Targets: Cholera
Impact Objectives: Increase global supply of affordable vaccine doses; deliver improved presentation at $1/dose
Status: IPO 2017 over 50 million doses to date
Intervention: Treatment
Targets: Onchocerciasis (river blindness)
Impact Objectives: Accelerate the eradication of onchocerciasis; improve treatment outcomes
Status: FDA approved 2018; Priority Review Voucher sold in 2019 to Novo Nordisk
GHIF Portfolio Recap